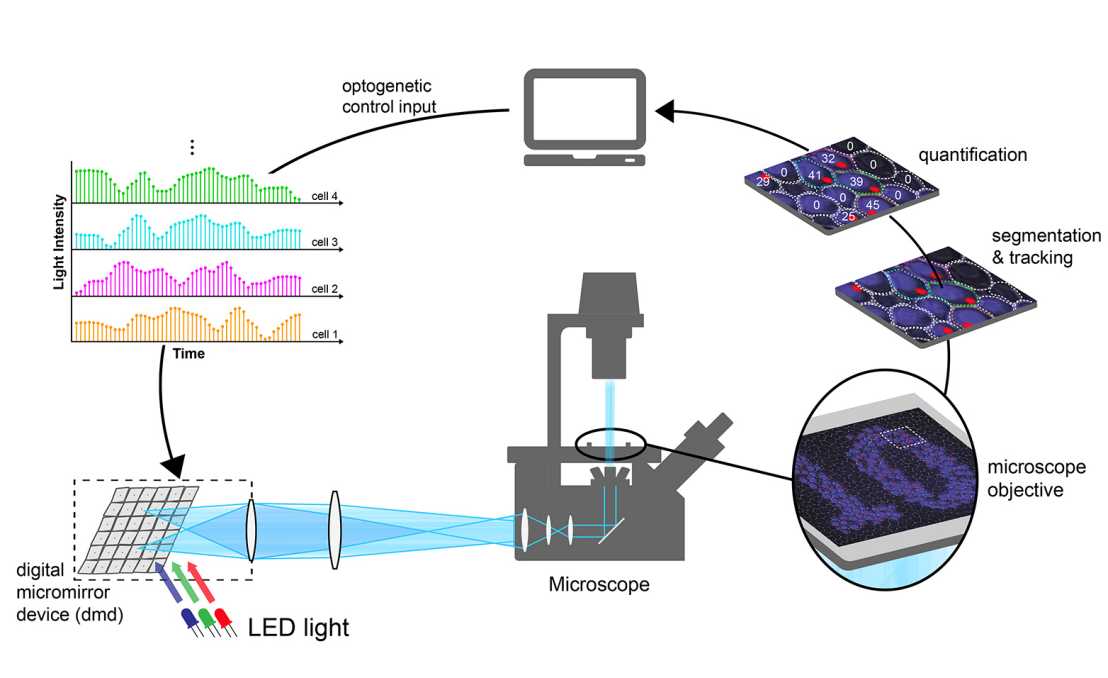
Principle of the Optogenetic Platform. (Graphic: M. Khammash/ETH Zurich)
The platform allows the researchers to illuminate single yeast cells with blue light for varying durations and intensities. Through the introduction of foreign genes, these cells have been genetically engineered to respond to light of this colour by activating a transcription factor and thereby promoting the transcription of a specific gene. The transcripts of this gene, the messenger RNAs, are tagged with fluorescent proteins, which results in bright fluorescent spots when transcription is actively occurring.
These spots can be imaged and evaluated using fluorescence microscopy. The researchers linked this system to a computer, which counts how many RNA molecules are transcribed at a given time and decides on the amount of light that each cell should receive next, in order to regulate their transcription as desired or as specified.
New insights on transcriptional dynamics
“Thanks to this set-up, we were able to show that transcriptional activation and deactivation happens very quickly,” says ETH Professor Khammash. His team further established that transcription occurs in bursts, whose duration and timing are modulated by transcription factor activity. Thanks to their feedback mechanism, the researchers were also able to reduce the differences in transcriptional output between different cells.
As a test (and to celebrate the 10th birthday of the Department for Biosystems last year), the researchers projected the number 10 onto yeast cells using blue light. In response to the light, transcription was initiated in the illuminated cells which resulted in the formation of bright fluorescent spots at the desired positions.
Dynamic signals reduce variability
The control method described has a limitation: it can only be used under the microscope, but not in test tubes or in bioreactors, where it would be needed to control biotechnological processes.
To overcome this limitation, the researchers turned to dynamic signalling. In contrast to conventional methods of controlling gene activity, the use of light allows cells to be controlled using complex, dynamic signals. By comparing different types of signals, the researchers observed in a study published in
Nature Communications
that individual cells react to pulsatile signals with higher accuracy than to constant signals. This now makes it possible to better control large populations of cells in a simple way. Furthermore, the results hint at a possible strategy by which variability may be regulated in natural cell populations.
Khammash is delighted that the projects were a success. He and his colleagues initially began this research out of pure curiosity, without any specific applications in mind. “We wanted, above all, to find out if we could control the random element of the transcription process,” he said. “It was a real technical challenge.”
He now thinks that his platform could be a gold mine for follow-up studies. He says that there could be many different applications, particularly in research, where controlling transcription is important. As an example, prototypes of genetic networks could be developed with increased speed, as cell-to-cell communication could be controlled externally and would no longer be reliant on signaling molecules. “The platform could also become an important tool for tissue engineering and stem cell research.” The researchers will take a closer look at these applications of their platform over the next few years, and have already secured funding from the EU FET open program to do so.